Actividad antiviral contra el SARS-CoV-2 empleando extractos de Ficus carica y Plectranthus amboinicus
Main Article Content
Abstract
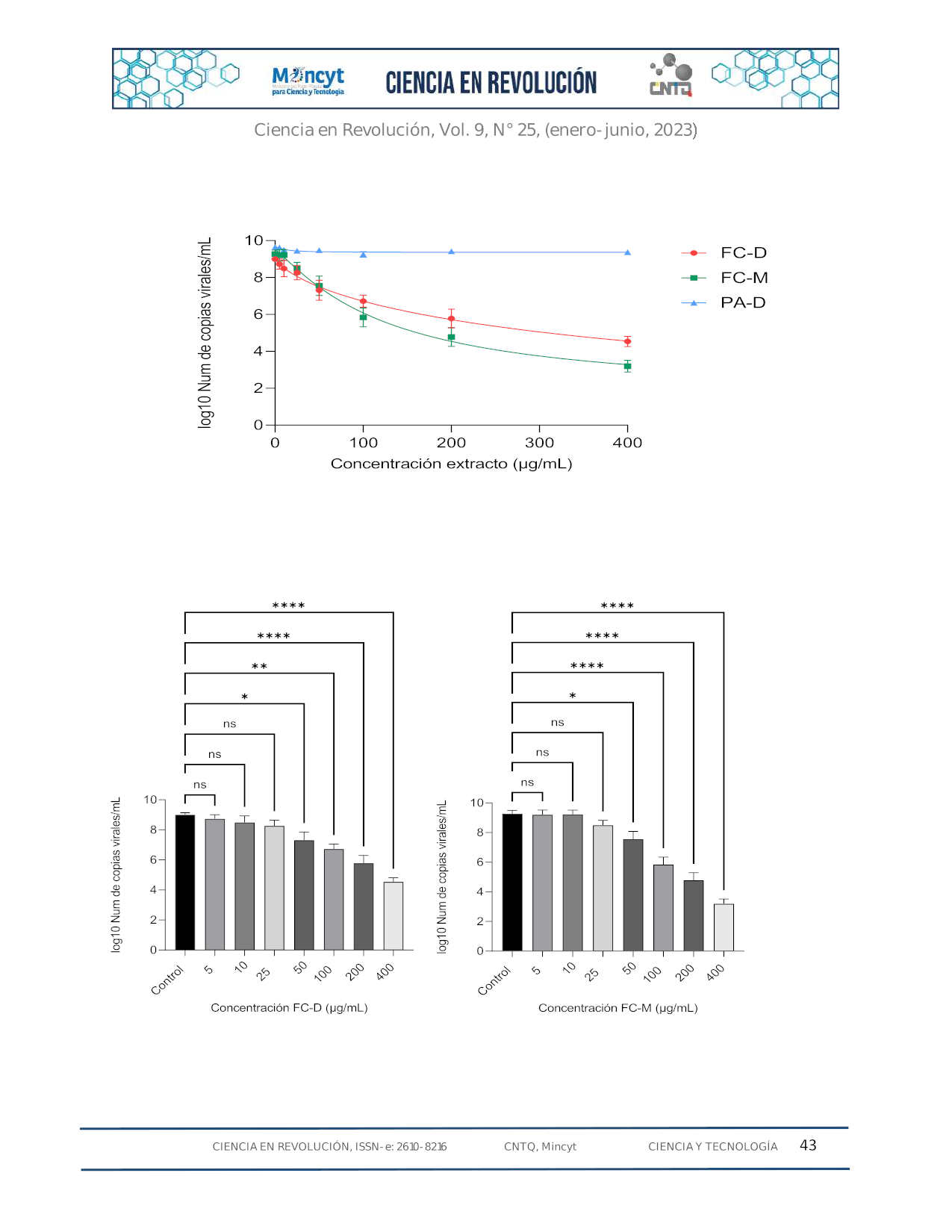
El SARS-CoV-2 es el responsable de la enfermedad COVID-19, que ha causado más de 765 millones de casos y 6,9 millones de muertes en todo el mundo. Hasta la fecha no se ha desarrollado un tratamiento específico. Una estrategia para diseñar medicamentos antivirales específicos contra el SARS-CoV-2 es inhibir enzimas claves implicadas en el ciclo viral, como las proteasas 3CLpro y PLpro. En este estudio se evaluó la capacidad de extractos seleccionados de las plantas de la higuera (Ficus carica) y el orégano orejón (Plectranthus amboinicus) para inhibir la infección de SARS-CoV-2 en células Vero E6. Los resultados mostraron que los extractos de la higuera inhibieron significativamente la replicación viral al reducir el número de copias virales determinadas mediante qRT-PCR y disminución en las unidades formadoras de placas mediante ensayos de reducción de placas líticas, mientras que los extractos de orégano orejón no mostraron una inhibición significativa en estos ensayos. Además, se evaluó el potencial viricida de los extractos de la higuera cuyos resultados sugieren que estos extractos podrían interactuar con la glicoproteína de la espiga evitando su internalización a la célula. En conclusión, los extractos de F. carica tienen considerable actividad antiviral, pudiendo ser consecuencia de la afectación de diferentes etapas del ciclo de replicación, lo que sugiere su potencial como fuente de compuestos antivirales contra el SARS-CoV-2.
Descargas
Article Details
References
Hu B, Guo H, Zhou P, Shi L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021. 19(3): 141-154. doi: 10.1038/s41579-
-00459-7.
Weekly epidemiological update on COVID-19 - 30 March 2023. Who.int. https://www.who.int/publications/m/item/weekly- epidemiological-update-on-covid-19 30-march-2023
Kim D, Lee J, Yang J, Kim J, Kim V, Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020. 181(4): 914-921.e10. doi: 10.1016/j.cell.2020.04.011.
Wang M, Zhao R, Gao L, Gao X, Wang D, Cao J. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10: 587269. doi: 10.3389/fcimb.2020.587269.
Hanson Q, Wilson K, Shen M, Itkin Z, Eastman R, Shinn P, Hall M. Targeting ACE2-RBD Interaction as a Platform for COVID-19 Therapeutics: Development and Drug-Repurposing Screen of an AlphaLISA Proximity Assay. ACS Pharmacol. Transl. Sci. 2020, (6): 1352- 1360. doi: 10.1021/acsptsci.0c00161.
Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85(9): 4122-34. doi: 10.1128/JVI.02232-10.
Hoffmann M, Kleine H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens T, Herrler G, Wu N, Nitsche A, Müller M, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020, 181(2): 271-280.e8. doi: 10.1016/j.cell.2020.02.052.
Yadav R, Chaudhary J, Jain N, Chaudhary P, Khanra S, Dhamija P, Sharma A, Kumar A, Handu S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells. 2021, 10(4): 821. doi: 10.3390/cells10040821.
Elshabrawy HA. SARS-CoV-2: An Update on Potential Antivirals in Light of SARS-CoV Antiviral Drug Discoveries. Vaccines (Basel). 2020, 8(2): 335. doi: 10.3390/vaccines8020335.
Center for Drug Evaluation, & Research. U.S. Food and Drug Administration; FDA. https://www.fda.gov/drugs/emergency- preparedness-drugs/coronavirus-covid-19-drugs
Organización Mundial para la Salud. WHO recommends highly successful COVID-19 therapy and calls for wide geographical distribution and transparency from originator. 2022.
https://www.who.int/news/item/22-04-2022-who-recommends- highly successful-covid-19-therapy-and-calls-for-wide-geographical- distribution-and-transparency-from-originator.
Santoro M, Carafoli E. Remdesivir: From Ebola to COVID-19. Biochem. Biophys. Res. Commun. 2021, 538: 145-150. doi: 10.1016/j.bbrc.2020.11.043.
Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, Leyssen P, Neyts J, Jochmans D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022, 198: 105252. doi: 10.1016/j.antiviral.2022.105252
Moyle G, Back D. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med. 2001, 2(2): 105-13. doi: 10.1046/j.1468-1293.2001.00063.x.
Lee C, Hsieh C, Ko W. Molnupiravir-A Novel Oral Anti-SARS-CoV-
Agent. Antibiotics (Basel). 2021, 10(11): 1294. doi: 10.3390/antibiotics10111294.
Fountain N, Vanhaeften R, Williamson J, Maskell J, Chua I, Charleston M, Cooley L. Antiviral treatments lead to the rapid accrual of hundreds of SARS-CoV-2 mutations in immunocompromised patients. Cold Spring Harbor Laboratory Press. 2022. doi: 10.1101/2022.12.21.22283811.
Conti V, Sellitto C, Torsiello M, Manzo V, De Bellis E, Stefanelli B, Bertini N, Costantino M, Maci C, Raschi E, Sabbatino F, Corbi G, Pagliano P, Filippelli A. Identification of Drug Interaction Adverse Events in Patients With COVID-19: A Systematic Review. JAMA Netw Open. 2022; 5(4):e227970. doi: 10.1001/jamanetworkopen.2022.7970.
Flynn J, Samant N, Schneider G, Barkan D, Yilmaz N, Schiffer C, Moquin S, Dovala D, Bolon D. Comprehensive fitness landscape of SARS-CoV-2 Mpro reveals insights into viral resistance mechanisms. Elife. 2022, 11:e77433. doi: 10.7554/eLife.77433.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J.
Immunol. Methods. 1983, 65(1-2): 55-63. doi: 10.1016/0022-
(83)90303-4.
Ortega J, Suárez A, Serrano M, Baptista J, Pujol F, Rangel H. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res. Ther. 2017. 14(1): 57. doi: 10.1186/s12981-017- 0183-6.
ISO 10993-5:2009. ISO. Biological evaluation of medical devices —
Part 5: Tests for in vitro cytotoxicity. 2022. https://www.iso.org/standard/36406.html. 2022.
Yarmolinsky L, Zaccai M, Ben-Shabat S, Mills D, Huleihel M. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. N. Biotechnol. 2009, 26(6): 307-13. doi: 10.1016/j.nbt.2009.08.005.
Houda Lazreg Aref. In vitro antiviral activities of Jrani caprifig latex and its related terpenes. Afr. J. Microbiol. Res. 2011;5(32). http://dx.doi.org/10.5897/ajmr10.104.
Baer A, Kehn-Hall K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J. Vis. Exp. 2014, (93):e52065. doi: 10.3791/52065.
Vaya J, Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). Biofactors. 2006, 28 (3-4): 169-75. doi: 10.1002/biof.5520280303.
Wu W, Li R, Li X, He J, Jiang S, Liu S, Yang J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses. 2015, 8(1):
doi: 10.3390/v8010006.
Mamouni K, Zhang S, Li X, Chen Y, Yang Y, Kim J, Bartlett M, Coleman I, Nelson P, Kucuk O, Wu D. A Novel Flavonoid Composition Targets Androgen Receptor Signaling and Inhibits Prostate Cancer Growth in Preclinical Models. Neoplasia. 2018, 20(8): 789-799. doi: 10.1016/j.neo.2018.06.003.
Ujjan ID, Khan S, Nigar R, Ahmed H, Ahmad S, Khan A. The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial. Front Nutr. 2023, 9: 1023997. doi: 10.3389/fnut.2022.1023997.
Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto
S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints.org. 2020. DOI: 10.20944/preprints202003.0226.v1.
Abdizadeh R, Hadizadeh F, Abdizadeh T. In silico analysis and identification of antiviral coumarin derivatives against 3- chymotrypsin-like main protease of the novel coronavirus SARS-CoV-
Mol. Divers. 2022, 26(2): 1053-1076. doi: 10.1007/s11030-021-10230-6.
Munafò F, Donati E, Brindani N, Ottonello G, Armirotti A, De Vivo
M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci. Rep. 2022, 12(1): 10571. doi: 10.1038/s41598-022-